How to Read & Interpret ISO/IEC 17025 Calibration Certificates
As an accreditation assessor, I can say that many common assessment deficiencies could have been prevented if the calibration certificate had been thoroughly reviewed.
This article is an adaptation of my popular presentation, “Beyond the Sticker & the Cert (Ensuring Better Measurements & Reducing Risk).” Product manufacturers, testing labs, and calibration labs, in attempts to reduce risk and ensure better measurements, will have their measuring and test equipment (M&TE) calibrated so they can have their “sticker and a cert.” But how often do people actually read their calibration certificates? How many know what to look for and how to correctly interpret and use the information? Are they simply checking a box or are they really ensuring better measurements and reducing risk? What disasters and recalls have occurred because one did not sufficiently review their calibration certificates? As an accreditation assessor, I can say that many common assessment deficiencies could have been prevented if the calibration certificate had been thoroughly reviewed.
The internationally recognized standard for calibration is ISO/IEC 17025:2017, General Requirements for the Competence of Testing and Calibration Laboratories. Among the requirements in this standard are the details to be reported on an ISO/IEC 17025-compliant calibration certificate. When an ISO/IEC 17025 calibration certificate is issued by an ISO/IEC 17025-accredited calibration lab, there are additional requirements dictated by the respective accreditation body and include propagations of ILAC (P14) ILAC Policy for Measurement Uncertainty in Calibration. For the purposes of this article, we will review the individual details of an ISO/IEC 17025-compliant calibration certificate and separately address the requirements on an ISO/IEC 17025-accredited calibration certificate.
To begin, an issued calibration certificate is a technical record. As a technical record, it must contain “sufficient information to facilitate, if possible, identification of factors affecting the measurement result and its associated measurement uncertainty and enable the repetition of the laboratory activity under conditions as close as possible to the original.”1
Reviewing one’s calibration certificate is a low-cost quality assurance activity. Think of the calibration certificate as telling a story about the equipment’s calibration. We are reviewing the Who, What, When, Where, and How. We will begin by assuming that we, as the consumer, understand how our measuring & test equipment (M&TE) is used (what parameters, functions, and ranges) and how accurate it needs to be.
Here is a numbered list of information required to be reported on an ISO/IEC 17025-compliant calibration certificate with explanations of each item:
“Title”2 is a description of what is this piece of paper (electronic or otherwise). It could be titled, “Calibration Certificate,” “Certificate of Calibration,” “Calibration Report,” or something similar. This is our first WHAT. It is the beginning detail to make sure we are reviewing a calibration certificate and not an invoice, packing slip, or something else.
“Name and address of the (calibration) laboratory”3 because we need to know who did the calibration and where they are located. This is a WHO. This is important because it should match to whom we sent our M&TE. Additionally, some labs have multiple locations. This also means that it must be clear as to the location of performance of the calibration activities4, whether at the calibration lab or at the customer’s location. The location data may be necessary for calculating corrections to the data for our location.
“Unique identifier”5 is something that differentiates one piece of M&TE’s calibration certificate from another calibration certificate for the same M&TE. This is a WHAT. This could be reported as a unique certificate number or could be based on a combination of details about the equipment calibrated, such as its job number + serial number + date. This identifier should appear on all pages of the respective certificate and there should be clear identification of the end of the reported certificate.
“Name and contact information of the customer”6 reflects who owns the M&TE; the other WHO. If you are buying the equipment through a vendor, that vendor may be listed as the M&TE “owner” because that is for whom the equipment was initially calibrated. I have also seen where someone received another company’s calibration certificate and filed it away without looking at it, only for the wrong customer to be sheepishly contacted by the calibration lab admitting to a breach of confidentiality.
“Identification of the method used”7 is a link of HOW the M&TE was calibrated. It could be a unique lab method identifier or reference to a standard method. Its intent is to link the calibration event to the method’s procedure and version so the measurements can be replicated.
“A description, unambiguous identification, and when necessary, the condition of the item”8 This is the WHAT, the information to help us identify the specific M&TE that was calibrated and that it is not for a different M&TE.
“The date of receipt of the calibration item(s), where this is critical to the validity and application of the results”9 This is a WHEN detail. Some M&TE needs to be conditioned in an environment for a time prior to calibrating it. In these cases, the date of receipt for calibration shall be reported.
“The date(s) of performance of the laboratory activity”10 This is WHEN the M&TE was calibrated.
If a due date or period of validity is assigned, it must either be dictated by the customer or if agreed upon by the customer. This is a WHEN. A calibration lab may not recommend the calibration interval on the certificate or label unless it has been agreed upon with the customer.11 The rationale for this prohibition is that the laboratory does not know the conditions of use of the M&TE, which may affect the length of time the calibration is valid. It may be appropriate for the customer to shorten or extend a calibration interval to best fit their needs and manage the risk of an out of tolerance “as found” condition at the next calibration. If an out of tolerance calibration occurs, the M&TE-owner must evaluate the impact of the out of tolerance impact on any affected data.
“The date of issue of the report”12 This is another WHEN. The issue date may be different than the date of calibration and should indicate the date the activity (certificate issuance) occurred.
“A statement to the effect that the results relate only to the items calibrated”13 This is a HOW. This statement is to prevent the usage of a calibration certificate to represent M&TE that is not listed on the certificate. Calibration is an activity for which one cannot sample a group of identical M&TE, calibrate one sampled M&TE, and then say that that one M&TE represents the calibration of all other M&TE in that group.
“The results with, where appropriate, the units of measurement”14 These are the calibration data; another WHAT. These data and units of measurement are critical to understanding how the M&TE performed during calibration. These are the data that we must review more closely and compare to our use requirements.
“Additions to, deviations, or exclusions from the method”15 These are a WHAT. If we are expecting calibration to a certain method and the calibration vendor deviates from the method, then we want to know this so we can appropriately understand and interpret the results. This is a nuanced difference that we will miss if we just look to make sure we have the calibration certificate versus reading the calibration certificate.
“Identification of the person(s) authorizing the report”16 This is a WHO. For the calibration lab performing the calibration, this is their evidence that they are confident in their measurements and that they are attesting the information and data on the report are valid.
“Clear identification when results are from external providers”17 This is another WHO. If you sent your M&TE to a calibration vendor and they are unable to calibrate it for you, then they must gain your approval before they subcontract the calibration to another calibration vendor.18 When you receive the calibration certificate, it must be clear from whom the results came.
“The measurement uncertainty of the measurement result presented in the same unit as that of the measurand or in a term relative to the measurand (e.g. percent)”19 This is a WHAT. There are two parts here; 1. A calibration without stated associated measurement uncertainty has broken the chain of its metrological traceability, and 2. The measurement result and its associated measurement uncertainty must be in the same or relatable units.
If the measurement result is reported in mm, the associated measurement uncertainty must also be in mm (or as a % or cm or m). It prohibited to mix units between the result & measurement uncertainty, such as where one is in “mm” and the other in “inch.”
“The conditions (e.g. environmental) under which the calibrations were made that have an influence on the measurement results”20 This is a HOW. Environmental conditions, such as temperature, barometric pressure, and local acceleration of gravity, can have significant impact on measurements and result in different values when you use the M&TE at a different location or in different conditions than for which it was calibrated.
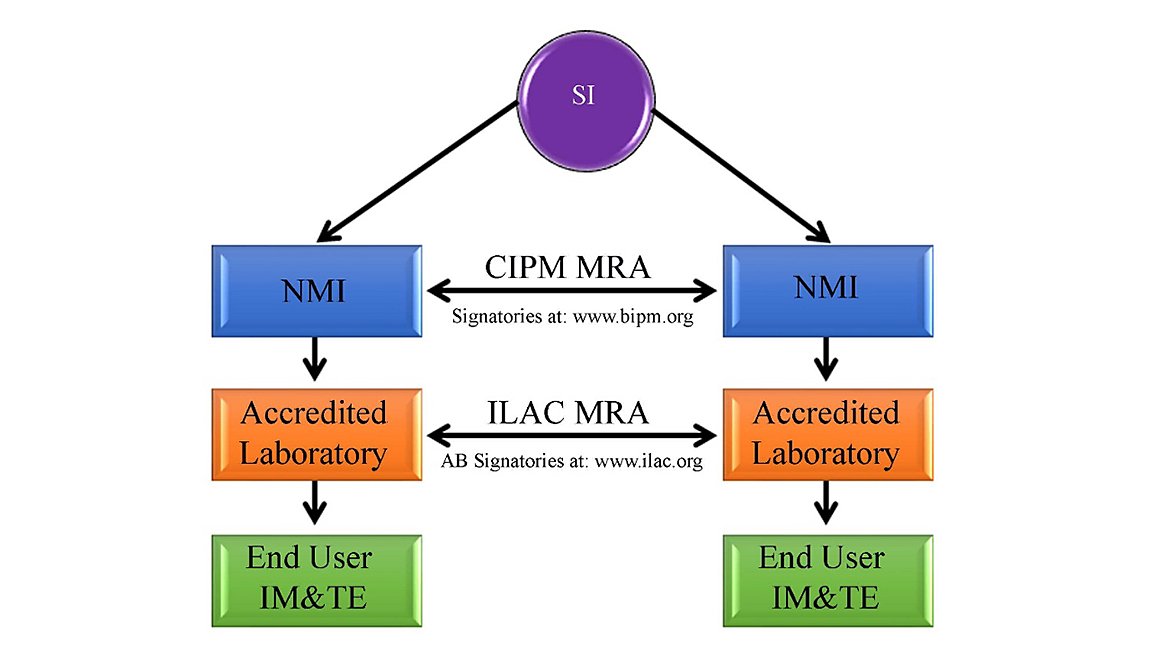
“A statement identifying how the measurements are metrologically traceable”21 This is a HOW. Metrological traceability is defined in the International Vocabulary of Metrology (VIM) as “property of a measurement result whereby the result can be related to a reference through a documented unbroken chain of calibrations, each contributing to the measurement uncertainty.”22 For this statement, many labs will state that their measurements are “NIST-traceable”. There is a lot to unpack about the misnomer, “NIST-traceable,” and that can be an article for another magazine edition. The more correct reference to measurement traceability is “measurement results are traceable to the International System of Units (SI)”23 A national metrology institute (NMI), even such as NIST, produce measurements that are traceable to the International System of Units (SI). If your unbroken chain of calibrations goes through another NMI than NIST, or is directly realized by the calibration lab, then using a statement saying their measurements are “NIST-traceable” is incorrect and you now have created a nonconformity.
A better statement of metrological traceability would be something like, ‘The item(s) on this certificate was calibrated with measurement standards traceable to SI Units through a CIPM-MRA signatory national metrological institute (NMI) such as NIST, certified reference materials, consensus standards, ratio methods, accepted intrinsic standards or fundamental physical constants.’
“The results before and after any adjustment or repair, if available”25 This is a WHAT. If you are using M&TE to make measurements, then the measurements must matter in some way. It is a great practice to have M&TE periodically calibrated to ensure your confidence in the measurements. Part of having confidence and defendable data is knowing how your M&TE performed prior to adjustments or repair, if available. (Sometimes M&TE breaks and calibration data before repair are not achievable.)
This is why the results before and after any adjustments or repair, if available, are critical to be reported.
By knowing the results before any adjustment or repair, you can know if your M&TE was in or out of tolerance (or somewhere in between). If you only request calibration data afteradjustments, where the M&TE is fully operational and able to make measurements, then you are assuming significant risk that your data are defendable. What other data have you collected that can defend that your M&TE was accurate since its last calibration?
By knowing the results after any adjustments or repair, if available, you now have a starting point for the M&TE accuracy in its current period of validity (aka, calibration interval).
“Where relevant, a statement of conformity with requirements or specifications”26 This is a HOW. This topic is an on-going challenge for M&TE users, calibration labs, testing labs, accreditation assessors, and accreditation bodies. The interpretation of what are acceptable applications of ‘Decision Rule’27 is an on-going discussion and debate. (Also see The Elephant in the Room, or the Impact of Measurement Uncertainty on Risk)
“Where appropriate, opinions and interpretations”28 This is a HOW. Opinions and interpretations on a calibration certificate are distinct from M&TE direct measurements and reporting of subsequent measurement data. There are ISO/IEC 17025 requirements regarding stating opinions and interpretations.
Any opinions or interpretations must be made by lab personnel who are authorized29. By limiting opinions and interpretations to be made by authorized personnel, the calibration lab reduces its risk of unauthorized personnel stating unfounded opinions & interpretations.
The lab must “document the basis upon which the opinions and interpretations have been made.”30 By having criteria upon which opinions and interpretations are made, there can be reproducibility in these opinions and interpretations.
Image from ASQ Metrology Handbook, 3rd Edition, Figure 9.1 Image Source: ASQ Metrology Handbook
ISO/IEC 17025 ACCREDITATION
When a calibration certificate is issued by an ISO/IEC 17025-accredited calibration lab, the requirements of ILAC P14 (as propagated by the respective Accreditation Body) also apply. Measurement uncertainty is a layered and nuanced topic beyond the scope of this article.
The ILAC Policy on Statement of Measurement Uncertainty on Calibration Certificates (ILAC P14:09:2020) includes the following numbered requirements.31 I have provided a brief explanation after each one.
Measurement uncertainty must be reported in compliance with the GUM.
For calibrations, the GUM (Guide to the Expression of Uncertainty in Measurement) is the guide for how measurement uncertainty is to be calculated and reported. The GUM also has guidance for reporting each of the steps of calculating the uncertainty
The measurement result shall include the measured quantity value y and the associated expanded uncertainty U.
A calibration measurement, y, without associated expanded measurement uncertainty, U, breaks the unbroken chain of metrological traceability; it is no longer a defendable calibration, and we cannot evaluate the measurement. The GUM provides the guidance of what the “expanded uncertainty U” represents and how it is calculated.
The coverage factor and the coverage probability shall be stated on the certificate, such as “ The reported expanded measurement uncertainty is stated as the standard measurement uncertainty multiplied by the coverage factor k such that the coverage probability corresponds to approximately 95 %.”
Here is a nuance about the coverage probability and its associated coverage factor, k; the measurement uncertainty is always estimated for (approximately) 95 % level of confidence and the corresponding coverage factor, k, is to achieve that 95 % level of coverage. Often this results in a k= 2, but sometimes k = 2.6 or something else than what can be rounded to 2. This is dependent on the overall degrees of freedom in the measurement.
The numerical value of the expanded uncertainty shall be given to, at most, two significant digits.
This is to provide uniformity and standardization in the expression of uncertainty on a calibration certificate.
Contributions to the uncertainty stated on the calibration certificate shall include relevant short-term contributions during calibration and contributions that can reasonably be attributed to the customer’s device.
It is critical that the device (M&TE) being calibrated is what is included in the relevant and reported calibration uncertainty for that device. Customers use their M&TE for many applications. For a customer to evaluate their M&TE’s associated calibration measurement uncertainty into their process, they must have a reasonable and relevant.
Accredited calibration laboratories shall not report a smaller measurement uncertainty than the uncertainty described by the CMC for which the laboratory is accredited.
The CMC, the “Calibration and Measurement Capability”32, is “a calibration and measurement capability available to customers under normal conditions.”33 Also see ILAC P14, Appendix A for further explanation of the term CMC.
As required in ISO/IEC 17025, accredited calibration laboratories shall present the measurement uncertainty in the same unit as that of the measurand or in a term relative to the measurand (e.g., percent).
This is to minimize errors from converting units as well as preventing use of incorrect uncertainty values because the measurement units do not correspond.
Now you have the information and some understanding of each of the required components of an ISO/IEC 17025 calibration certificate. The good news is that once you start reviewing your calibration certificates, it becomes much easier and more efficient. Perhaps create your own checklist of what to review as a record that you have reviewed the certificate. Too many times as customers we simply look for the “Pass” or “Fail” on the certificate and then throw it in a file cabinet. We must look beyond the simple statement of “Pass” or “Fail” or that we even received a calibration certificate and look atall the information and data on our calibration certificates. By going “Beyond the Sticker and the Cert,” we may be able to prevent some catastrophic failures and embarrassing recalls due to inaccurate or uncalibrated M&TE. Applying this knowledge and practicing the skills of reviewing the calibration certificates is a way to ensure better measurements and reduce risk.